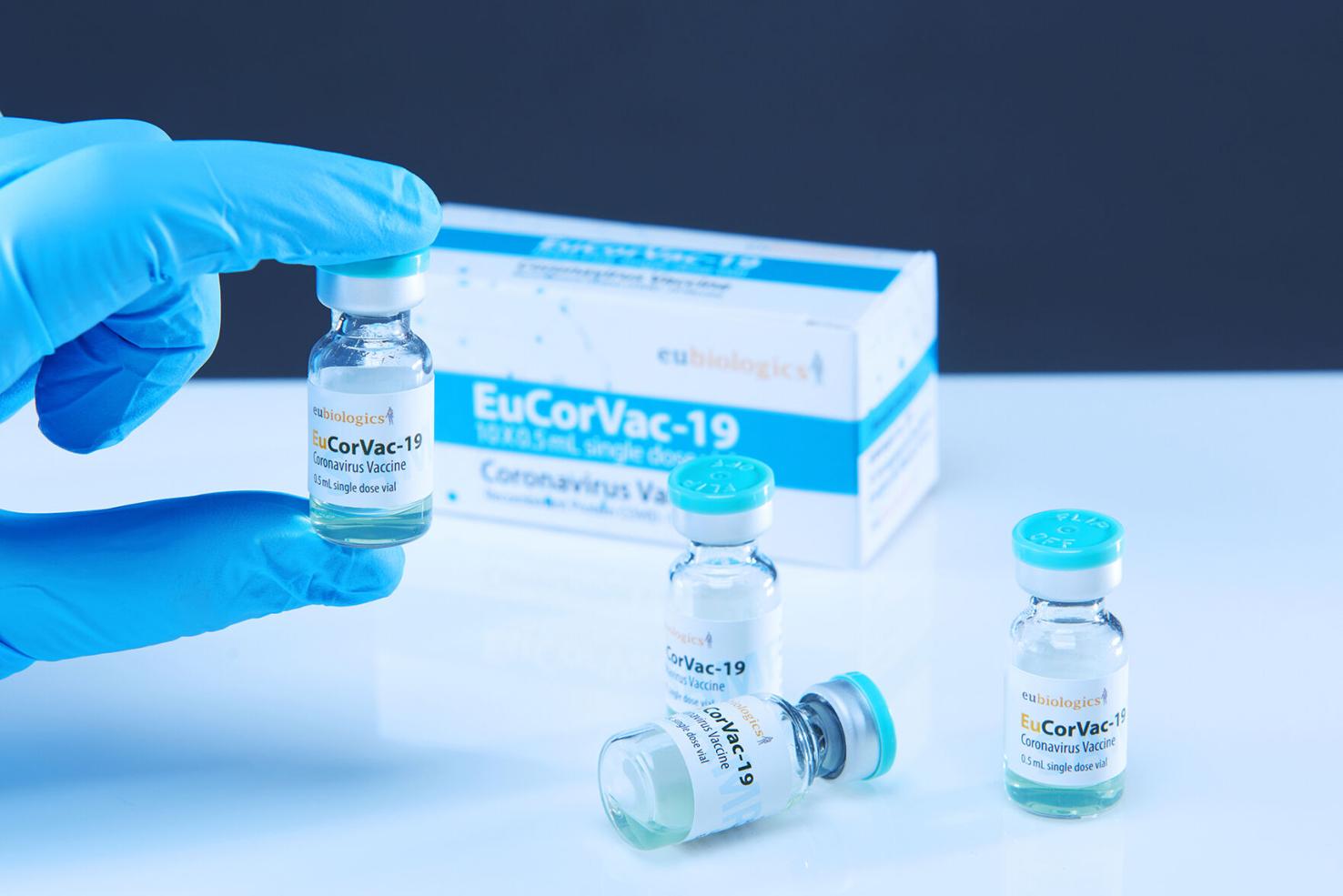
POP Biotechnologies’ next-gen vaccine technology to enter COVID-19 phase III clinical trials
February 4, 2022 – POP Biotechnologies (POP BIO), a Buffalo, NY based biopharmaceutical startup, announces the approval of the plan for a phase 3 clinical study of EuCorVac-19, a COVID-19 vaccine candidate developed by South Korean partner EuBiologics (KOSDAQ: 206650i) from the Ministry of Food and Drug Safety in South Korea. EuCorVac-19, is a protein-based vaccine consisting of Eubiologics protein antigen, EulMT, and POP BIO’s spontaneous-nanoliposome antigen particle (SNAP) vaccine technology. SNAP enables proteins to present on the surface of nanoliposomes, resulting in improved immune responses. Dr. Jonathan Lovell, co-founder of POP BIO said “The goal of the large scale clinical testing of this next-generation technology is to add a new vaccine tool to the arsenal for combatting COVID-19”.
Eubiologics will soon commence a Phase III comparison trial for 4,000 healthy participants in the Philippines, Bangladesh and other countries based on a standard comparison clinical trial guideline. Eubiologics will work closely with international research organizations for expedited recruitment in countries with low vaccination rates as well as the analysis of trial results to accelerate the regulatory approval and commercialization of EuCorVac-19.
EuCorVac-19 has already proved its safety and efficacy in a Phase I/II clinical trial, the results of which were announced in December 2021. Eubiologics expects to expedite the development of Omicron variant vaccine based on the same platform used for EuCorVac-19.
About POP Biotechnologies:
POP Biotechnologies, Inc. is a privately held biotechnology company focused on the research and development of novel therapeutics and vaccines employing their proprietary porphyrin-phospholipid (PoP) liposome technologies. The PoP technology, exclusively licensed from the State University of New York Research Foundation was developed at The State University of New York at Buffalo (SUNY Buffalo).
About POP BIO’s SNAP Technology:
POP BIO’s Spontaneous Nanoliposome Antigen Particleization (SNAP) technology enables the rapid development and manufacturing of highly immunogenic particle-based vaccines and immunotherapies directed against infectious disease and cancer through the use of a cobalt modified variant of the PoP technology (CoPoP). The SNAP technology enables rapid and stable particle-formation and liposome-display of protein and peptide antigens resulting in substantial improvements in immune responses. POP BIO is currently working with several other pharmaceutical partners to evaluate the SNAP technology.
About Eubiologics:
Eubiologics is a South Korean Biotechnology company that received funds for the Phase I/II EuCorVac-19 trial from the new drug development committee of the Ministry of Health and Welfare in Korea and recently submitted the application for phase III trial to get additional financial assistance. Eubiologics has 2 main animal-based bioreactors (1,000L) to produce recombinant protein antigens at Chuncheon Plant 1 and EcML which is adjuvant at Chuncheon Plant 2. The total capacity of COVID-19 vaccine is from 100M to 200M doses per year. EuCorVac-19 vaccine uses the same type of cell line, which is for antibody treatment, so if the company uses these facilities, its manufacturing capacity can be further expanded.
Media Contact
Company Name: POP BIO
Contact Person: Jonathan Smyth
Email: Send Email
City: Buffalo
State: NY
Country: United States
Website: http://pop.bio